药物临床试验数据递交PMDA的规定
药物临床试验数据递交PMDA的规定
信息来源:
https://www.pmda.go.jp/files/000247157.pdf
Technical Conformance Guide on Electronic Study Data Submissions April 1, 2022 Provisional Translation (as of June 2022) *(电子研究数据提交的技术一致性指南)
仅提取该文档中的部分内容加以翻译,以下中文都是机器翻译,仅供自学
3.4 File size of electronic study data 电子研究数据的文件大小
Of electronic study data to be submitted, PDF files must not exceed the maximum file size of PDF specified in the eCTD v4 notification. An applicant should consult the PMDA beforehand if the total file size in a single operation and/or the size of each file of electronic study data other than PDF files exceed the upper limit. For the upper limit for the file size, please refer to the operation manual for the gateway system.
对于要提交的电子研究数据,PDF文件不得超过eCTD v4通知中规定的PDF的最大文件大小。如果单个操作中的总文件大小和/或除PDF文件以外的每个电子研究数据文件的大小超过上限,申请人应事先咨询PMDA。有关文件大小的上限,请参阅网关系统的操作手册。
3.5 Folder structure 文件夹结构
In principle, electronic study data should be submitted after storing it in the following folder structure, and no additional subfolders should be created. If storing the data in the following folder structure is difficult, the applicant must consult the PMDA beforehand and make a submission after agreeing on the folder structure and the storage of files. Hierarchies down to “m5¥datasets¥[study id / iss / ise]”cannot be changed.
原则上,电子研究数据在存储后应按以下文件夹结构提交,不创建其他子文件夹。如果难以在以下文件夹结构中存储数据,申请人必须事先咨询PMDA,并在同意文件夹结构和文件存储后提交。等级制度低至“m5¥datasets¥[study id / iss / ise]”无法更改。

Note the following points when storing electronic study data into the abovementioned folder structure.
将电子研究数据存储到上述文件夹结构中时,请注意以下几点。
The path length counting from the “m5” folder, including the file name, must be 160 characters or shorter.
从“m5”文件夹开始计数的路径长度(包括文件名)必须为 160 个字符或更短。
Folder names should be 32 characters or fewer, and must be comprised of the following characters.
Alphabetic characters from “a” to “z” [U+0061 to U+007A];
Numbers from “0” to “9” [U+0030 to U+0039];
Low line “_” [U+005F];
Hyphen-minus “-” [U+002D];
文件夹名称应为 32 个字符或更少,并且必须由以下字符组成。
从“a”到“z”的字母字符 [U+0061 到 U+007A];
从 “0” 到 “9” 的数字 [U+0030 到 U+0039];
低行 “_” [U+005F];
连字符减号 “-” [U+002D];
File names should be 32 characters or fewer for datasets and 64 characters or fewer for files other than datasets (including the extension), and the name excluding the dot and the extension must be comprised of the following characters. For characters to be used for file names, please refer to the operation manual for the gateway system.
Alphabetic characters from “a” to “z” [U+0061 to U+007A];
Numbers from “0” to “9” [U+0030 to U+0039];
Low line “_” [U+005F];
Hyphen-minus “-” [U+002D];
对于数据集,文件名应为 32 个字符或更少,对于数据集以外的文件(包括扩展名),文件名应为 64 个字符或更少,并且不包括点和扩展名的名称必须由以下字符组成。有关用于文件名的字符,请参阅网关系统的操作手册。
从“a”到“z”的字母字符 [U+0061 到 U+007A];
从 “0” 到 “9” 的数字 [U+0030 到 U+0039];
低行 “_” [U+005F];
连字符减号 “-” [U+002D];
The reviewer’s guide, define.xml, and style sheet must be stored in the same folder as their corresponding datasets. The style sheet should display the information in the define.xml, which is to be submitted to the PMDA.
审阅者指南、define.xml和样式表必须与相应的数据集存储在同一文件夹中。样式表应显示要提交到 PMDA 的 define.xml中的信息。
If there is no file to store the data, this folder should not be created.
如果没有用于存储数据的文件,则不应创建此文件夹。
When storing electronic study data in the field of clinical pharmacology in a format other than the CDISC standard, one example is to create and store it in a “cp” folder under the “analysis” folder as mentioned in the table below. In such a case, there will be no special restriction on the folder structure within the “cp” folder. The method of storing the data is not limited to that mentioned above. However, regardless of the method of storage, the above considerations on path length, folder name, and file name must be followed. It is preferable to consult the PMDA beforehand on the method of storing the data.
当以CDISC标准以外的格式存储临床药理学领域的电子研究数据时,一个例子是将其创建并存储在“分析”文件夹下的“cp”文件夹中,如下表所述。在这种情况下,对“cp”文件夹中的文件夹结构没有特殊限制。存储数据的方法不限于上述方法。但是,无论使用哪种存储方法,都必须遵循上述有关路径长度、文件夹名称和文件名的注意事项。最好事先咨询PMDA关于数据存储的方法。
The usage method of each folder is as follows.
每个文件夹的使用方法如下。

3.6 Validation of electronic study data 电子研究数据的验证
Electronic study data, which are submitted via the gateway system, will be validated according to the type of data. The results of the validation will be notified to the applicant via the gateway system. Electronic study data, which violate the rules shown in 3.6.1 (a), should be corrected prior to the submission of a new drug application and, preferably, all data should be resubmitted. If an error occurs during data transmission or while operating the gateway system, please contact the gateway system help desk for directions.
通过网关系统提交的电子研究数据将根据数据类型进行验证。验证结果将通过网关系统通知申请人。违反3.6.1(a)所示规则的电子研究数据应在提交新药申请之前进行更正,最好是重新提交所有数据。如果在数据传输期间或操作网关系统时发生错误,请联系网关系统帮助台以获取指示。
3.6.1 Validation of data conforming to the CDISC standards 验证符合CDISC标准的数据
The PMDA will perform validation of data that conform to the CDISC standards using Pinnacle 21 Enterprise
PMDA将使用Pinnacle 21 Enterprise对符合CDISC标准的数据进行验证
The rules used for validation have been classified by the level of importance taking into consideration the characteristics of each rule and based on conformity to the standards, ease of use of the data in review, quality of the clinical study data which the PMDA should know beforehand, and future uses of the clinical study data by the PMDA. The levels of importance are shown below.
用于验证的规则已根据重要性级别进行分类,同时考虑到每个规则的特征,并基于是否符合标准,审查数据的易用性,PMDA应事先了解的临床研究数据的质量以及PMDA未来对临床研究数据的使用。重要性级别如下所示。
(a) Rules which, if violated, will cause the review to be suspended until corrections have been made Very basic rules such as the presence/absence of necessary datasets for each clinical study
(a)如果违反,将导致审查暂停的规则,直到做出更正非常基本的规则,例如每个临床研究是否存在必要的数据集
(b) Rules which, if violated, will require an explanation In many cases, these rules are clearly stated in each standard and implementation guide, and if violated, the applicant should explain in the reviewer’s guide about the reason for the violation and the reason why it is not possible to correct it.
(b) 如果违反这些规则,则需要解释 在许多情况下,这些规则在每个标准和实施指南中都有明确规定,如果违反,申请人应在审查员指南中解释违规的原因和无法纠正的原因。
(c) Rules which, even when violated, will not necessarily require any explanation The reason for the violation possibly is requested separately for the above (c) from the perspective of the quality of the clinical study data.
(c)规则,即使违反,也不一定需要任何解释从临床研究数据的质量的角度来看,上述(c)可能单独要求违反的原因。
Details of the environment in which the PMDA performs validations, individual rules and their importance are published on the PMDA’s website. Before submitting electronic study data, the applicant should perform a validation, and should take the necessary action for any violations that are identified. Please keep in mind that these rules may be revised and that validation should always be performed after confirming the latest information. If any rules are revised, the content of the revision will be available for a certain period before the revised rules are applied.
PMDA执行验证的环境、单个规则及其重要性的详细信息发布在PMDA的网站上。在提交电子研究数据之前,申请人应进行验证,并应对发现的任何违规行为采取必要的措施。请记住,这些规则可能会被修改,并且应在确认最新信息后始终执行验证。如果任何规则被修订,修订的内容将在修订后的规则适用之前的一段时间内可用。
4. Electronic study data to be submitted 提交电子研究数据
4.1 CDISC-conformant electronic study data and relevant documents 符合CDISC标准的电子研究数据及相关文件
4.1.1 Datasets to be submitted 提交数据集
4.1.1.1 Overview 概述
CDISC standards should be used for the submission of clinical study data. For the latest standards and implementation guide (IG) of CDISC and the development status, please refer to the CDISC’s website (https://www.cdisc.org/).
CDISC标准提交临床研究数据时应采用。有关CDISC的最新标准和实施指南(IG)以及发展现状,请参阅CDISC的网站(https://www.cdisc.org/)。
The CDISC provides SDTM as the standard for datasets comprising clinical study data collected from the CRF and other records in a tabulation format and ADaM as the standard for analysis datasets. Data should be summarized using the designated variables and submitted in accordance with the versions of SDTM and ADaM standards and IG that are accepted by the PMDA.
CDISC提供SDTM作为数据集的标准,包括从CRF和其他记录中以表格格式收集的临床研究数据,以及ADaM作为分析数据集的标准。应使用指定的变量对数据进行汇总,并根据PMDA接受的SDTM和ADaM标准以及IG版本提交数据。
To avoid the complication of having to convert the collected data into a CDISC-conformant format upon application, it is preferable to decide on the method of data collection and analysis procedures that will conform to these standards from the planning stage of the clinical study. However, these standards do not indicate the items of the clinical study data required for the review. Therefore, the items of data to be collected from each clinical study must be established, which enables efficacy and safety evaluations of the drug and ensures the subjects’ safety, by taking into consideration the characteristics of the target disease and the drug. If there are multiple methods of implementation based on the clinical study design or characteristics of the data to be collected or if some aspects of the dataset cannot or may not conform to the standards or IG upon creating a dataset conforming to these standards, the applicant should consult the PMDA beforehand.
为了避免在应用时必须将收集的数据转换为符合CDISC的格式的复杂性,最好从临床研究的规划阶段决定符合这些标准的数据收集和分析程序的方法。然而,这些标准并未表明评价所需的临床研究数据项目。因此,必须确定从每个临床研究中收集的数据项,通过考虑目标疾病和药物的特征,可以对药物进行疗效和安全性评估并确保受试者的安全性。如果基于临床研究设计或要收集的数据的特征有多种实施方法,或者如果数据集的某些方面在创建符合这些标准的数据集时不能或可能不符合标准或IG,申请人应事先咨询PMDA。
CDISC standards, controlled terminologies to be used, and terms defined in dictionaries should be used without modifying spelling or notation, such as capital and small letters, when creating datasets.
创建数据集时,应使用 CDISC 标准、要使用的受控术语以及字典中定义的术语,而不修改拼写或表示法(如大写字母和小写字母)。
4.1.1.2 SDTM datasets SDTM数据集
The SDTM dataset is to be submitted, after the data collected from the CRF and other records are stored into the domain using variables designated by the version of SDTM and SDTM IG. The applicant may manage the clinical study data using their own unique format that includes SDTM, but even in such cases, the dataset to be submitted must be converted into formats that are in accordance with SDTM and SDTM IG.
SDTM数据集在使用SDTM和SDTM IG版本指定的变量存储到域中后,从通用报告格式和其他记录收集的数据将提交。申请人可以使用自己独特的格式(包括SDTM)管理临床研究数据,但即使在这种情况下,要提交的数据集也必须转换为符合SDTM和SDTM IG的格式。
In SDTM, the variables are classified into Required, Expected, and Permissible. If the variables contain collected data, the data for all variables must be submitted to the extent possible, regardless of the classification. Of the Permissible and Expected variables, the following must be submitted to the extent possible.
在 SDTM 中,变量分为必需、预期和允许。如果变量包含收集的数据,则无论分类如何,都必须尽可能提交所有变量的数据。在允许变量和预期变量中,必须尽可能提交以下内容。
Baseline flags (such as laboratory results, vital signs, ECG, pharmacokinetic concentration, and microbiology results)
基线标志(如实验室结果、生命体征、心电图、药代动力学浓度和微生物学结果)
EPOCH designator (variables that designate the period)
EPOCH 指示符(指定周期的变量)
If the dataset contains --DTC, --STDTC, and --ENDTC variables, the corresponding Study Day variables (--DY, --STDY, and --ENDY)
如果数据集包含 --DTC、--STDTC 和 --ENDTC 变量,则相应的研究日变量 (--DY、--STDY 和 --ENDY)
SDTM dataset stores the data obtained in the study and does not include values used to impute missing data. Imputed data should be included in the ADaM dataset.
SDTM 数据集存储研究中获得的数据,不包括用于插补缺失数据的值。估算数据应包含在 ADaM 数据集中。
Basic rules of SDTM should be followed, for example, dates must be in accordance with ISO 8601 format, --DY must not contain 0, and even when the data was collected as Yes/No in the CRF, it must be stored as Y/N in SDTM dataset.
应遵循SDTM的基本规则,例如,日期必须符合ISO 8601格式,--DY不得包含0,即使数据在CRF中收集为Yes/No,也必须在SDTM数据集中存储为Y / N。
USUBJID has been prepared as a variable for storing the unique ID assigned to each individual subject across the entire application. It enables the data of one subject in multiple studies, for example, in a phase III study and the subsequent long-term study, to be summarized. Therefore, consideration should be given to ensure that each subject has the same USUBJID across all the studies in the application to the extent possible.
USUBJID已准备为一个变量,用于存储分配给整个应用程序中的每个主题的唯一ID。它能够总结多个研究中一个受试者的数据,例如,在III期研究和随后的长期研究中。因此,应考虑确保每个受试者在申请中的所有研究中都具有相同的USUBJID。
In SDTM, the domains of the Trial Design Model, such as TS domain, store information on the designs of clinical studies and thus contain useful information on the characteristics of the clinical studies. Its data should, therefore, be stored in accordance with the IG to the extent possible and be submitted.
在SDTM中,试验设计模型的领域(例如TS领域)存储有关临床研究设计的信息,因此包含有关临床研究特征的有用信息。因此,其数据应尽可能按照IG进行存储并提交。
In SDTM, it is possible to set a series of datasets called SUPPQUAL to include variables that are not specified in SDTM. These datasets may be used for data that cannot be allocated to each of the domains of SDTM. However, basically, variables related to main analyses should not be included in such datasets. If an applicant is considering to include a variable which is not mentioned specifically in the IG but may be important for the review in the SUPPQUAL, the applicant should consult the PMDA beforehand, and if the variable has been included in the SUPPQUAL, this should preferably be explained in the reviewer’s guide.
在 SDTM 中,可以设置一系列名为 SUPPQUAL 的数据集,以包含 SDTM 中未指定的变量。这些数据集可用于无法分配给 SDTM 的每个域的数据。但是,基本上,与主要分析相关的变量不应包含在此类数据集中。如果申请人正在考虑包括IG中未特别提及但可能对SUPPQUAL中的审查很重要的变量,申请人应事先咨询PMDA,如果该变量已包含在SUPPQUAL中,则最好在审查员指南中对此进行解释。
Depending on the characteristics of the collected data, it may not fit into an existing domain of SDTM. In such a case, it is acceptable for the applicant to create a custom domain. To perform this, the applicant must confirm that the data does not fit into existing domains, then create a custom domain according to the SDTM IG, and store the data under this domain. Explanation of the custom domain, together with the reason why it was necessary, should be described in the reviewer’s guide.
根据所收集数据的特征,它可能不适合SDTM的现有领域。在这种情况下,申请人可以创建自定义域。为此,申请人必须确认数据不适合现有域,然后根据SDTM IG创建自定义域,并将数据存储在此域下。自定义域的解释以及必要原因应在审阅者的指南中进行描述。
4.1.1.3 ADaM datasets ADaM数据集
The analysis datasets should be submitted after they are constructed in accordance with ADaM. It is not necessary to submit ADaM datasets for all analyses described in the statistical analysis plan. However, ADaM datasets should be submitted for analyses performed to obtain important results on efficacy and safety and clinical study results that provide the rationales for setting of the dosage and administration, such as primary efficacy analysis and secondary efficacy analyses (secondary analyses of primary variable and analyses of key secondary variables), primary safety analyses and basic analyses of adverse events, and analyses to investigate the effect of major factors on efficacy and safety. The applicant should preferably consult the PMDA beforehand on the sufficiency of the datasets to be submitted. Even when the analysis results in the clinical study report had been created using datasets other than ADaM datasets, for analyses related to important results on efficacy and safety and the rationales for setting of the dosage and administration mentioned above, the analysis datasets based on ADaM that can reproduce these results should be submitted. It is not necessary to submit additional analysis datasets for analyses that the applicant performed after the application in response to inquiries.
分析数据集应按照ADaM构建后提交。没有必要为统计分析计划中描述的所有分析提交 ADaM 数据集。然而,应提交ADaM数据集进行分析,以获得关于疗效和安全性的重要结果,以及为确定剂量和给药提供依据的临床研究结果,例如一级疗效分析和二级疗效分析(一级变量的二次分析和关键次级变量的分析),初级安全性和不良事件的基本分析, 并进行分析,以调查主要因素对功效和安全性的影响。申请人最好事先咨询PMDA,了解要提交的数据集的充分性。即使临床研究报告中的分析结果是使用ADaM数据集以外的数据集创建的,对于与功效和安全性的重要结果以及上述剂量和给药设置的基本原理相关的分析,也应提交基于ADaM的分析数据集,以重现这些结果。对于申请人在申请后为回应查询而执行的分析,无需提交其他分析数据集。
In ADaM, there is a dataset called Analysis Data Subject Level (ADSL), which contains subject level information, and this must be submitted for each study for which the ADaM datasets have been submitted. To make each analysis using the ADaM dataset easier, the core variables, including all covariates described in the protocol that are generally included in the ADSL dataset, in principle, should be included in each ADaM dataset. Examples of such variables include covariates, study (protocol number), study site (site number), region, country, assigned treatment, sex, age, race, analysis population flags, and other important baseline demographic variables.
在 ADaM 中,有一个名为分析数据主体级别 (ADSL) 的数据集,其中包含主题级别信息,必须为已提交 ADaM 数据集的每个研究提交此信息。为了使使用 ADaM 数据集的每次分析更加容易,原则上应将核心变量(包括协议中描述的通常包含在 ADSL 数据集中的所有协变量)包含在每个 ADaM 数据集中。此类变量的示例包括协变量、研究(方案编号)、研究地点(站点编号)、区域、国家/地区、指定的治疗、性别、年龄、种族、分析人口标志和其他重要的基线人口统计变量。
Please keep in mind that ADaM datasets other than ADSL can consist of various variables depending on the nature of the individual target analysis, and it is, therefore, important to explain the content of these datasets in the definition document and the reviewer’s guide.
请记住,ADSL以外的ADaM数据集可以由各种变量组成,具体取决于各个目标分析的性质,因此,在定义文档和审稿人指南中解释这些数据集的内容非常重要。
If the variables used in the SDTM dataset are also used in the ADaM dataset, these variables must have the same attributes and content.
如果 SDTM 数据集中使用的变量也在 ADaM 数据集中使用,则这些变量必须具有相同的属性和内容。
4.1.1.4 File formats of datasets 数据集的文件格式
The SDTM and ADaM datasets that conform to the CDISC standards should be submitted in the SAS XPORT file transport format Version 5 (hereinafter referred to as SAS XPORT format), which is the data transport format released by the SAS Institute, and as one file per dataset. The SAS CPORT Procedure must not be used when creating XPORT transport files by the SAS system. Similarly, for datasets that contain variables written in Japanese because it was considered necessary and appropriate (hereinafter referred to as Japanese items), the files should be in the SAS XPORT format, and the character sets or the encoding scheme used to create the dataset should be described in the reviewer’s guide.
符合CDISC标准的SDTM和ADaM数据集应以SAS XPORT文件传输格式版本5(以下简称SAS XPORT格式)提交,这是SAS研究所发布的数据传输格式,每个数据集一个文件。在 SAS 系统创建 XPORT 传输文件时,不得使用 SAS CPORT 过程。同样,对于包含日语编写的变量的数据集(因为日语项目被认为是必要和适当的),文件应采用 SAS XPORT 格式,并且用于创建数据集的字符集或编码方案应在审阅者指南中进行描述。
The file name and the dataset name must be the same for the SDTM and ADaM datasets.
对于 SDTM 和 ADaM 数据集,文件名和数据集名称必须相同。
4.1.2 Definition documents and other appended documents of datasets 数据集的定义文档和其他附加文档
4.1.2.1 Definition documents of datasets 数据集的定义文档
The definition documents of the SDTM and ADaM datasets in the DefineXML format by the CDISC should respectively be created into the XML format files containing references to the style sheets that enable their contents to be displayed and stored in the same folder as their corresponding datasets, together with these style sheets. The file name of the definition document should be “define.xml”. The definition document should include the definitions of datasets, variables, possible values of variables, and controlled terminologies and codes. The information on controlled terminologies and dictionaries should include their versions
CDISC的SDTM和ADaM数据集的定义文档应分别创建为XML格式文件,其中包含对样式表的引用,使其内容能够显示并存储在与其对应数据集相同的文件夹中, 与这些样式表一起。定义文档的文件名应为“define.xml”。定义文档应包括数据集、变量、变量的可能值以及受控术语和代码的定义。关于受控术语和词典的信息应包括其版本
In order for the review of clinical study data to progress smoothly, it is important that the relationship between the analysis results shown in the application documents and the analysis datasets is easily understandable. Therefore, the definition documents of the ADaM datasets should preferably include Analysis Results Metadata, which shows the relationship between the analysis results and the corresponding analysis dataset and the variables used, for the analyses performed to obtain the main results of efficacy and safety and clinical study results that provide the rationales for setting of the dosage and administration,shown in 4.1.1.3. The Analysis Results Metadata of each analysis should preferably include the following items.
为了使临床研究数据的审查顺利进行,重要的是申请文件中显示的分析结果与分析数据集之间的关系易于理解。因此,ADaM数据集的定义文档最好包括分析结果元数据(ARM=Analysis Result Metada),其显示分析结果与相应分析数据集和所用变量之间的关系,以便进行分析以获得疗效和安全性的主要结果以及临床研究结果,为剂量和给药的设定提供依据,如 4.1.1.3 所示。每个分析的分析结果元数据最好包括以下项目。
Figure or table numbers and titles showing the analysis results displayed in the clinical study report
显示临床研究报告中显示的分析结果的图或表号和标题
Purpose and reasons for performing the analysis
执行分析的目的和原因
Parameter name and code to be used
要使用的参数名称和代码
Variables subject to analysis
有待分析的变量
Dataset to be used
要使用的数据集
Selection criteria for the records subject to analysis
待分析记录的选择标准
Corresponding description in the statistical analysis plan, analysis program name, and summary of the analytical methods
统计分析方案中的对应说明、分析程序名称、分析方法摘要
Extract of the analysis program corresponding to the analysis method
分析方法对应的分析程序摘录
For the format of the Analysis Results Metadata, the applicant should refer to the Analysis Results Metadata Specification for Define-XML by the CDISC to the extent possible, but if it is difficult to include it into the definition document, it is possible to submit it as a separated file in PDF format, as specified in the eCTD v4 notification. The explanations in the definition document may be written in Japanese.
对于分析结果元数据的格式,申请人应尽可能参考 CDISC 的 Define-XML 分析结果元数据规范,但如果难以将其包含在定义文档中,则可以将其作为 PDF 格式的单独文件提交,如 eCTD v4 通知中所述。定义文件中的解释可以用日语编写。
4.1.2.2 Annotated CRF 注释CRF (acrf)
The Annotated CRF shows the relationship between each item of data collected from the CRF and the variables included in the dataset. For datasets that conform to the CDISC standards, SDTM variables will be used as variables that correspond to the items in the CRF. For the method of annotating, please refer to the SDTM Metadata Submission Guidelines (SDTM-MSG) by the CDISC.
acrf式显示了从CRF收集的每一项数据与数据集中所列变量之间的关系。对于符合 CDISC 标准的数据集,SDTM 变量将用作与 CRF 中的项目相对应的变量。有关注释方法,请参阅 CDISC 的 SDTM 元数据提交指南 (SDTM-MSG)。
Data collected from the CRF should preferably be stored in the SDTM dataset whenever possible, prior to submission. However, if there are items of data that are not going to be submitted, it should be made clear in the Annotated CRF that the data is not included in the submitted dataset, and the reason why this data was not included should be provided in the reviewer’s guide. However, it is not necessary to perform this if the reasons are obvious
从CRF收集的数据最好在提交之前尽可能存储在SDTM数据集中。但是,如果有一些数据项目不打算提交,则应在acrf中明确指出,该数据未包括在提交的数据集中,并且应在审评员指南中提供未包括这些数据的原因。但是,如果原因很明显,则没有必要执行此操作。
The file format of the Annotated CRF, in principle, should be a PDF, as specified in the eCTD v4 notification, and the file name should be “acrf.pdf”. In principle, it should be stored in the same folder as SDTM datasets.
原则上,acrf应为PDF格式,如eCTD v4通知中所述,文件名应为“acrf.pdf”。原则上,它应该存储在与SDTM数据集相同的文件夹中。
4.1.2.3 Reviewer’s guide 审阅指南(RG)
To promote the understanding of the content and characteristics of the datasets by reviewers during the review and enable the applicant to explain about the utilization status of and conformance to the data standards when creating the datasets, a dataset definition document as well as a reviewer’s guide must be created for each of the SDTM and ADaM datasets, which, in principle, should be stored in the same folder as their corresponding datasets prior to submission.
为了促进审稿人在审评过程中对数据集内容和特征的理解,使申请人在创建数据集时能够解释数据标准的利用状况和符合性,必须为每个SDTM和ADaM数据集创建数据集定义文档和审评者指南, 原则上,在提交之前,它们应存储在与其相应数据集相同的文件夹中。
In principle, the following items should be included in the reviewer’s guide for the SDTM dataset.
原则上,SDTM 数据集的审阅者指南中应包含以下项目。
Clinical study name, protocol number
临床研究名称、方案编号
Explanation of the clinical study design
临床研究设计说明
Standards, controlled terminologies, and dictionaries used when creating datasets and their versions (SDTM, SDTM IG, SDTM Controlled Terminology, Define-XML, MedDRA, and WHODrug Global)
创建数据集及其版本时使用的标准、受控术语和字典(SDTM、SDTM IG、SDTM 受控术语、Define-XML、MedDRA 和 WHODrug Global)
Explanation of the annotated CRF
对acrf的解释
List of datasets to be submitted
要提交的数据集列表
SDTM datasets of the trial design
试验设计的SDTM数据集
SDTM datasets of the subject data (including information about custom domains, SUPP, and use of Japanese in the datasets and SUPP)
受试者数据的 SDTM 数据集(包括有关自定义域、SUPP 以及在数据集和 SUPP 中使用日语的信息)
Other datasets to be submitted
其他递交的数据集
Explanation of the subject data (including explanation of custom domains)
受试者数据的说明(包括自定义域的说明)
Information on conformance to the data standards
有关符合数据标准的信息
Validation tool used for the validation and its version
用于验证及其版本的验证工具
Version of the validation rules used for the validation
用于验证的验证规则的版本
Explanation on conformance to the data standards (explanation of the validation results including the identification number and importance of a rule with a detected violation)
关于是否符合数据标准的说明(验证结果的说明,包括检测到违规行为的规则的标识号和重要性)
In principle, the following items should be included in the reviewer’s guide for the ADaM dataset.
原则上,以下项目应包含在 ADaM 数据集的审阅者指南中。
Clinical study name, protocol number
研究名称、实验方案编号
Explanation of the clinical study design related to the analysis datasets
与分析数据集相关的临床研究设计说明
Standards, controlled terminologies, and dictionaries used when creating datasets and their versions (ADaM, ADaM IG, ADaM Controlled Terminology, Define-XML, MedDRA, and WHODrug Global)
创建数据集及其版本时使用的标准、受控术语和字典(ADaM、ADaM IG、ADaM Controlled Terminology、Define-XML、MedDRA 和 WHODrug Global)
Considerations related to multiple analysis datasets
与多个分析数据集相关的注意事项
Considerations on creating the analysis datasets
创建分析数据集的注意事项
List of datasets to be submitted
要提交的数据集列表
ADaM datasets (including information that uses Japanese)
ADaM 数据集(包括使用日语的信息)
Other datasets to be submitted
要提交的其他数据集
Explanation of the datasets
数据集说明
Information on conformance to the data standards
有关符合数据标准的信息
Validation tool used for the validation and its version
用于验证及其版本的验证工具
Version of the validation rules used for the validation
用于验证的验证规则的版本
Explanation on conformance to the data standards (explanation of the validation results including the identification number and importance of a rule with a detected violation)
关于是否符合数据标准的说明(验证结果的说明,包括检测到违规行为的规则的标识号和重要性)
Information on the program
程序的信息
Analysis environment and software used
使用的分析环境和软件
Explanation of programs that were used to create the ADaM datasets to be submitted and programs for analyses (if they cannot be submitted, specifications that show the analysis algorithm) to be submitted
用于创建要提交的 ADaM 数据集的程序以及要提交的分析程序(如果无法提交,则说明显示分析算法的规范)
When creating reviewer’s guides for the SDTM and ADaM datasets, although no specific format for the reviewer’s guide is provided as the CDISC standards, materials that the applicant may refer to are published.
在为SDTM和ADaM数据集RG时,尽管没有RG的特定格式作为CDISC标准,但申请人可以参考的材料已经发布。
Each document should in principle be created as a PDF, as specified in the eCTD v4 notification, and the files for SDTM and ADaM should preferably be named “study-data-reviewers-guide.pdf” or “csdrg.pdf”, “analysis-datareviewers-guide.pdf” or “adrg.pdf”, or the like, so that their contents are identifiable. The reviewer’s guide may be written in Japanese.
原则上,每个文档应创建为PDF格式,如eCTD v4通知中所述,SDTM和ADaM的文件最好命名为“study-data-reviewers-guide.pdf”或“csdrg.pdf”,“analysis-datareviewers-guide.pdf”或“adrg.pdf”等,以便其内容可识别。审稿人指南可以用日语撰写。
4.1.3 Version of standards to be used 要使用的标准版本
When creating the dataset and the definition document conforming to the CDISC standards, please refer to the PMDA’s website for versions of the CDISC standards, controlled terminologies, and dictionaries that are accepted by the PMDA. Acceptance of versions is judged based on the date of submission described in the new drug application by the applicant.
创建符合 CDISC 标准的数据集和定义文档时,请参阅 PMDA 的网站,了解 PMDA 接受的 CDISC 标准版本、受控术语和字典。版本的接受是根据申请人在新药申请中描述的提交日期来判断的。
It is sufficient to use different versions within the same application, but the same version must be used within the same clinical study. If the applicant had referred to other versions for certain domains within the same clinical study, the version used and the reason for using that version must be explained in the reviewer’s guide.
在同一应用程序中使用不同的版本就足够了,但在同一临床研究中必须使用相同的版本。如果申请人在同一临床研究中引用了某些领域的其他版本,则必须在审稿人指南中解释所使用的版本以及使用该版本的原因。
Datasets of integrated analyses of multiple clinical studies should be created using the same version, even if the version used to create the dataset of each clinical study was different. When standardizing the version, the reason that the specific version was chosen and considerations for converting to a different version should be described in the reviewer’s guide. In cases where the version standardization is difficult and different versions are unavoidably referred to in the same analysis, describe in the reviewer’s guide whether the difference between versions used affects the datasets to be submitted and, if it affects the datasets, detail the difference in the reviewer’s guide.
应使用相同的版本创建多个临床研究的综合分析数据集,即使用于创建每个临床研究数据集的版本不同。在标准化版本时,应在审阅者指南中描述选择特定版本的原因以及转换为其他版本的注意事项。如果版本标准化很困难,并且在同一分析中不可避免地引用了不同的版本,请在审阅者指南中说明所用版本之间的差异是否会影响要提交的数据集,如果影响数据集,则在审阅者指南中详细说明差异。
4.1.4 Therapeutic area standards 治疗领域标准
To date, the Therapeutic Area Standards have been published for a number of diseases by the CDISC for storing data specific to each therapeutic area. These standards may be used for diseases for which standards have already been published. However, the standards used must be provided in the definition document of the datasets and the reviewer’s guide.
迄今为止,CDISC已经发布了针对许多疾病的治疗领域标准,用于存储特定于每个治疗领域的数据。这些标准可用于已经发布标准的疾病。但是,所使用的标准必须在数据集的定义文档和审稿人指南中提供。
4.1.5 Handling of data written in Japanese text 处理用日文文本编写的数据
If variables had been collected in Japanese and there is a risk of losing certain information by translating it into English, the descriptions in Japanese are necessary and appropriate, and data written in Japanese (hereinafter referred to as Japanese data) may be submitted. Examples of variables that may contain Japanese texts are shown in Attachment 2 (but are not limited to these).
如果用日文收集了变量,并且有将某些信息翻译成英文而丢失某些信息的风险,则日文说明是必要和适当的,可以用日文写成的数据(以下简称日语数据)提交。可能包含日语文本的变量示例如附件 2 所示(但不限于这些变量)。
The method of storing Japanese data into datasets and the method of submission when a domain contains Japanese items, in principle, will be as follows. Examples are provided in Attachment 3.
原则上,将日语数据存储到数据集中的方法以及域包含日语项目时的提交方法如下。附件3提供了示例。
For the domain (dataset), two datasets: the Japanese dataset and a dataset comprising letter sets specified by ASCII such as alphanumeric characters (hereinafter referred to as alphanumeric dataset) should be created.
对于域(数据集),应创建两个数据集:日语数据集和包含由 ASCII 指定的字母集(如字母数字字符)的数据集(以下简称字母数字数据集)。
In the Japanese dataset, only the Japanese items should be Japanese and the rest should be alphanumeric data, similar to that in the alphanumeric dataset.
在日语数据集中,只有日语项应该是日语,其余项目应该是字母数字数据,类似于字母数字数据集中的数字数据。
The Japanese dataset and alphanumeric dataset must be identical in structure, except for the data lengths of the Japanese items and the corresponding alphanumeric character sequence, and the two datasets must also have an identical record number and record order. The applicant only needs to submit the definition document for the alphanumeric dataset, and the definition document must be stored in the same folder as alphanumeric datasets.
日语数据集和字母数字数据集在结构上必须相同,除了日语项的数据长度和相应的字母数字字符序列外,两个数据集还必须具有相同的记录编号和记录顺序。申请人只需提交字母数字数据集的定义文档,定义文档必须与字母数字数据集存储在同一文件夹中。
In the alphanumeric dataset, an English character sequence (such as “JAPANESE TEXT IN SOURCE DATA”) which clearly states this is not the original data should be stored in the parts that correspond to the Japanese items. If the Japanese data must be stored by multiple variables or records due to restriction on the data length, this English character sequence must be stored in the corresponding records within the alphanumeric dataset.
在字母数字数据集中,明确说明这不是原始数据的英文字符序列(如“JAPANESE TEXT IN SOURCE DATA”)应存储在与日文项目相对应的部分。如果由于数据长度的限制,日语数据必须由多个变量或记录存储,则此英文字符序列必须存储在字母数字数据集内的相应记录中。
These English character sequences (such as “JAPANESE TEXT IN SOURCE DATABASE”) must be consistent within the same study, and it must be stated clearly in the reviewer’s guide or the definition document (define.xml) of the dataset.
这些英文字符序列(例如“JAPANESE TEXT IN SOURCE DATABASE”)必须在同一研究中保持一致,并且必须在数据集的审稿人指南或定义文档(define.xml)中明确说明。
For parts that correspond to the questionnaires and code lists that contain Japanese text, an appropriate English translation or English character sequence must be stored. When storing English character sequences and distinguishing each of the character sequences, appropriate measures such as by attaching a number at the end of each sequence (example: “JAPANESE TEXT IN SOURCE DATABASE 01” and “JAPANESE TEXT IN SOURCE DATABASE 02”) should be taken, and the link with the original Japanese text should be shown in the reviewer’s guide.
对于与包含日语文本的调查表和代码列表相对应的部分,必须存储相应的英文翻译或英文字符序列。在存储英文字符序列并区分每个字符序列时,应采取适当的措施,例如在每个序列的末尾附加一个数字(例如:“JAPANESE TEXT IN SOURCE DATABASE 01”和“JAPANESE TEXT IN SOURCE DATABASE 02”),并且应在审阅者指南中显示与原始日语文本的链接。
Alphanumeric datasets must be included in the designated folder. The Japanese dataset corresponding to the SDTM dataset must be included in the “sdtm_j” folder, and the Japanese dataset corresponding to the ADaM dataset must be included in the “adam_j” folder. The same dataset name and label name must be used for the two datasets.
字母数字数据集必须包含在指定的文件夹中。与SDTM数据集对应的日文数据集必须包含在“sdtm_j”文件夹中,而与ADaM数据集对应的日文数据集必须包含在“adam_j”文件夹中。必须对这两个数据集使用相同的数据集名称和标签名称。
For domains that do not contain Japanese items, only the alphanumeric dataset must be included in the designated folder, and the submitted data must not contain any duplicates.
对于不包含日语项目的域,指定文件夹中只能包含字母数字数据集,并且提交的数据不得包含任何重复项。
In principle, for Japanese data, both SDTM and ADaM datasets should be stored by the above method before they are submitted, but if the applicant plans to submit data from clinical studies containing Japanese data, it is preferable to consult the PMDA beforehand on the scope of such data.
原则上,对于日本数据,SDTM和ADaM数据集在提交之前应通过上述方法存储,但如果申请人计划提交包含日本数据的临床研究数据,则最好事先咨询PMDA关于此类数据的范围。
4.1.6 Submission of programs 程序的递交
4.1.6.1 Programs to be submitted 准备递交的程序
With respect to the programs related to electronic study data conforming to the CDISC standards, the programs used to create the ADaM datasets and programs used for analyses must be submitted for the analyses performed to obtain the important results on efficacy and safety and clinical study results that provide the rationales for setting of the dosage and administration shown in 4.1.1.3. The main purposes of requesting the submission of these programs are to understand the process by which the variables for the analyses were created and to confirm the analysis algorithms. Therefore, it is not necessary to submit the programs in a format or content that allows the PMDA to directly run the program under its given environment. Also, although the programs to be submitted are not limited to specific software or versions, information on the environment in which the programs were created or run (operation system and software used and their versions) must be provided together in the reviewer’s guide. If programs with macros had been used, the macro programs should preferably be submitted together. However, if submission of the macro program is difficult or submission of the program itself is difficult because the creation of the dataset or program was outsourced, the submission of specifications that show the analysis algorithm would be sufficient.
对于符合CDISC标准的电子研究数据相关程序,必须提交用于创建ADaM数据集的程序和用于分析的程序,以获得关于疗效和安全性的重要结果以及临床研究结果,这些结果为4.1.1.3所示剂量和给药的设定提供了基本原理。请求提交这些程序的主要目的是了解创建分析变量的过程并确认分析算法。因此,没有必要以允许PMDA在其给定环境下直接运行程序的格式或内容提交程序。此外,尽管要提交的程序不限于特定的软件或版本,但有关创建或运行程序的环境(操作系统和所使用的软件及其版本)的信息必须在审阅者指南中一起提供。如果使用了带有宏的程序,则最好一起提交宏程序。但是,如果提交宏程序很困难,或者由于数据集或程序的创建是外包的,因此程序本身的提交很困难,则提交显示分析算法的规范就足够了。
4.1.6.2 File format of the programs 程序的文件格式
As stated in 4.1.6.1, although the programs to be submitted are not limited to specific software or versions, the file name should include the extension attached by the analysis software. If the file name does not contain an extension, an explanation about the file format should be included in the reviewer’s guide.
如4.1.6.1所述,虽然要提交的程序不限于特定的软件或版本,但文件名应包括分析软件附带的扩展名。如果文件名不包含扩展名,则应在审阅者指南中包含有关文件格式的说明。
4.1.7 CDISC-conformant electronic study data on clinical pharmacology analyses 临床药理学分析CDISC符合电子研究数据
For the handling of CDISC-conformant electronic study data on clinical pharmacology analyses, please refer to 4.1.1 to 4.1.6. Points to be considered especially for those data are described in this section.
关于临床药理学分析的CDISC符合标准的电子研究数据的处理,请参阅4.1.1至4.1.6。本节将特别针对这些数据考虑要点。
4.1.7.1 SDTM datasets SDTM数据集
PP domain storing pharmacokinetic parameters should be submitted as well as PC domain storing drug concentration data, since the pharmacokinetic parameters themselves are considered as data to capture the characteristics of the drug. Creating RELREC dataset based on SDTM IG is preferable when explaining the relationship of datasets between PC domain and PP domain. However, it would be also acceptable to explain those relationship in a document such as the reviewer’s guide if it is difficult to create the RELREC dataset based on SDTM IG.
PP结构域应提交存储药代动力学参数以及PC结构域存储药物浓度数据,因为药代动力学参数本身被视为捕获药物特性的数据。在解释PC域和PP域之间的数据集关系时,基于SDTM IG创建RELREC数据集是优选的。但是,如果很难基于SDTM IG创建RELREC数据集,则在文档(例如审稿人指南)中解释这些关系也是可以接受的。
In the case where PC domain and PP domain are created by converting from datasets in a format other than CDISC standards, the traceability (the procedure of creating the PC domain and PP domain, the relationship between the information included in these domains, such as the relationship between the variables in PC domain and those in PP domain, etc.) should be explained in a document such as the reviewer’s guide. In this case, PK parameters will not need to be recalculated from the PC domain for the purpose of the explanation of the traceability
在PC域和PP域是通过以CDISC标准以外的格式转换数据集来创建的的情况下,可追溯性(创建PC域和PP域的过程,这些域中包含的信息之间的关系,例如PC域中的变量与PP域中的变量之间的关系, 等)应在文档(如审稿人指南)中进行解释。在这种情况下,出于可追溯性解释的目的,不需要从PC域重新计算PK参数
4.1.7.2 ADaM datasets ADaM数据集
When ADaM datasets for PK or PK/PD analysis of blood and urine drug concentration are submitted, either a single dataset or multiple datasets would be acceptable as long as each analysis using the ADaM dataset can be performed easily without modification of the dataset.
当提交用于血液和尿液药物浓度的PK或PK / PD分析的ADaM数据集时,只要使用ADaM数据集的每次分析都可以轻松执行而无需修改数据集,就可以接受单个数据集或多个数据集。
When the datasets used for the analysis are in a format other than ADaM and are converted to ADaM datasets for the application, it is not necessary to submit the original datasets used for the analysis. However, if the original datasets are useful for explanation of traceability between datasets or contents of analysis, it may be submitted and used for the explanation.
当用于分析的数据集采用 ADaM 以外的格式并转换为应用程序的 ADaM 数据集时,无需提交用于分析的原始数据集。但是,如果原始数据集对于解释数据集之间的可追溯性或分析内容有用,则可以提交并用于解释。
4.2 Electronic study data on clinical pharmacology analyses 关于临床药理学分析的电子研究数据
Matters that need to be followed with respect to the specific contents of the submission, including the “Explanation of electronic study data package on clinical pharmacology”, as well as electronic study data on clinical pharmacology and programs are shown below.
提交的具体内容需要遵循的事项,包括“临床药理学电子研究数据包的说明”,以及关于临床药理学和程序的电子研究数据如下所示。
4.2.1 “Explanation of electronic study data package on clinical pharmacology” “临床药理学电子研究数据包的解释”
Since electronic study data on clinical pharmacology are stored in various folders and various file names, the PMDA will use the “Explanation of electronic study data package on clinical pharmacology” to review and use electronic study data submitted. “Explanation of electronic study data package on clinical pharmacology” is created by the PMDA based on the information about content of the clinical pharmacology study data provided via the gateway system when submitting electronic study data or described in the XML message of the eCTD. Since information entered in the analysis type are used to identify files on clinical pharmacology when creating the “Explanation of electronic study data package on clinical pharmacology”, the analysis type should be appropriately entered.
由于临床药理学的电子研究数据存储在各种文件夹和各种文件名中,PMDA将使用“临床药理学电子研究数据包的说明”来审查和使用提交的电子研究数据。“临床药理学电子研究数据包的解释”是由PMDA根据提交电子研究数据时通过网关系统提供的临床药理学研究数据内容的信息或在eCTD的XML消息中描述的信息创建的。由于在创建“临床药理学电子研究数据包说明”时,在分析类型中输入的信息用于识别临床药理学文件,因此应适当输入分析类型。
In case of submissions using the PMDA window due to inevitable reasons, TSV files may be submitted, except for when referencing electronic study data from the XML message of the eCTD.
如果由于不可避免的原因使用PMDA窗口提交,则可以提交TSV文件,除非引用eCTD的XML消息中的电子研究数据。
Information used to create the “Explanation of electronic study data package on clinical pharmacology” by the PMDA is as follows:
PMDA用于创建“临床药理学电子研究数据包的解释”的信息如下:
Study ID: Letter sets identifying each study
研究 ID:标识每个研究的字母集
File path: A file path from the m5 folder, from which information on the file name is also obtained. In case of submissions of electronic study data using the gateway system, entering the file path is not necessary, except for when referencing electronic study data from the XML message of the eCTD because the gateway system imports the file path automatically.
文件路径:来自 m5 文件夹的文件路径,从中还可以获得有关文件名的信息。如果使用网关系统提交电子研究数据,则无需输入文件路径,除非从eCTD的XML消息中引用电子研究数据,因为网关系统会自动导入文件路径。
Analysis type: Type of analysis performed [Enter STS (standard pharmacokinetic analysis), POP (population analysis), PBPK (physiologically based pharmacokinetic model analysis), or Other (other than the above) using abbreviation]. For clinical study data that are not electronic study data on clinical pharmacology (refer to Attachment 1), enter Non-CP for all files. STS includes pharmacokinetic/pharmacodynamic analysis performed by the same method as for standard pharmacokinetic analysis. Enter Other for clinical pharmacology studies or analyses that are not STS, POP, or PBPK.
分析类型:执行的分析类型 [使用缩写输入 STS(标准药代动力学分析)、POP(总体分析)、PBPK(基于生理学的药代动力学模型分析)或其他(上述分析除外)。]对于不是临床药理学电子研究数据的临床研究数据(请参阅附件1),请为所有文件输入Non-CP。STS 包括通过与标准药代动力学分析相同的方法执行的药代动力学/药效学分析。输入其他用于临床药理学研究或非 STS、POP 或 PBPK 的分析。
Description: Explanation of the file content, which is used when STS, POP, PBPK, or Other is designated for the analysis type. Details need to be described specifying the use of each file and its relationship to other files. Description should be 100 characters or less.
说明:文件内容的说明,在为分析类型指定 STS、POP、PBPK 或“其他”时使用该文件内容。需要描述详细信息,指定每个文件的用途及其与其他文件的关系。说明应为 100 个字符或更少。
4.2.2 Specific content of electronic study data to be submitted 提交的电子研究数据的具体内容
Matters that must be followed with respect to standard pharmacokinetic analysis, population analysis, and physiologically based pharmacokinetic model analysis are shown below.
标准药代动力学分析、群体分析、生理药代动力学模型分析等注意事项如下图所示。
When submitting electronic study data on clinical pharmacology analyses that conform to the CDISC standards, please refer to 4.1. However, even in this case, please refer to 4.2.2.1 (3) for the analysis specifications on pharmacokinetics or pharmacokinetics/pharmacodynamics and information to be submitted in accordance with these specifications.
提交符合CDISC标准的临床药理学分析电子研究数据时,请参阅4.1。但是,即使在这种情况下,有关药代动力学或药代动力学/药效学的分析规范以及根据这些规范提交的信息,请参阅4.2.2.1(3)。
4.2.2.1 Standard pharmacokinetic analysis 标准药代动力学分析
Details in the case where analysis datasets are submitted in a format other than ADaM for the submission of electronic study data on standard pharmacokinetic analysis are as follows:
以ADaM以外的格式提交分析数据集以提交标准药代动力学分析电子研究数据的情况如下:
(略)
4.2.2.2 Population analysis (including simulations) 人群分析(包括模拟)
Details for the submission of electronic study data on population analysis are as follows:
提交关于人群分析的电子研究数据的细节如下:
(1) Analysis dataset 分析数据集
Analysis dataset, which is finally used for model building of base model and final model, etc., and corresponds to program files for the model, should be submitted. The file format should be as shown below, but an optional file format other than the followings is acceptable:
SAS XPORT format (*.xpt)
ASCII Format Data Files
应提交分析数据集,该数据集最终用于基础模型和最终模型等的模型构建,并与模型的程序文件相对应。文件格式应如下所示,但可接受以下可选文件格式:
SAS XPORT 格式 (*.xpt)
ASCII 格式数据文件
(2) Dataset definition documents Refer to 4.2.2.1(2)
数据集定义文档,参见4.2.2.1 (2)
(3) Program files 程序文件
Program files for analysis using base model and final model, etc., should be submitted. The file format should be as shown below, but an optional file format other than the followings is acceptable: ASCII Format Data Files
应提交使用基础模型和最终模型等进行分析的程序文件。文件格式应如下所示,但可接受以下可选文件格式: ASCII 格式数据文件
(4) Files into which major results are outputted 应提交主要结果输出到的文件
Output files including the analysis output using base model and final model, etc., (such as NONMEM output) should be submitted. The file format is optional.
输出文件,包括使用基本模型和最终模型的分析输出等,(如NONMEM输出)。文件格式是可选的。
(5) Files related to simulation 与模拟相关的文件
If the simulation results are used for decision-making, such as selection of patients to treat and setting of the dosage and administration, files related to the simulation should be submitted. The simulation results in this case include results on model-based posterior estimates, such as posterior estimates of exposure-related parameters (AUC, Cmax, etc.). As files related to the simulation, program files for generating simulation data, performing simulation and making figures and tables showing simulation results etc. should be submitted. Submission of files related to a simulation performed for model evaluation (such as visual predictive check) and datasets created in the course of simulation is not mandatory. If a dataset, etc. related to existing information is used in the simulation, the dataset, etc. including such information, should be submitted. If it is difficult to submit those programs, the submission of specifications that show the analysis algorithm would be sufficient. The file format of programs is optional.
如果模拟结果用于决策,例如选择要治疗的患者以及剂量和给药的设置,则应提交与模拟相关的文件。在这种情况下,模拟结果包括基于模型的后验估计结果,例如暴露相关参数(AUC,Cmax等)的后验估计值。作为与仿真相关的文件,应提交用于生成仿真数据、进行仿真、制作显示仿真结果的图形和表格等的程序文件。提交与为模型评估(例如视觉预测检查)而执行的模拟相关的文件以及在模拟过程中创建的数据集不是强制性的。如果在模拟中使用与现有信息相关的数据集等,则应提交包含此类信息的数据集等。如果提交这些程序很困难,则提交显示分析算法的规范就足够了。程序的文件格式是可选的。
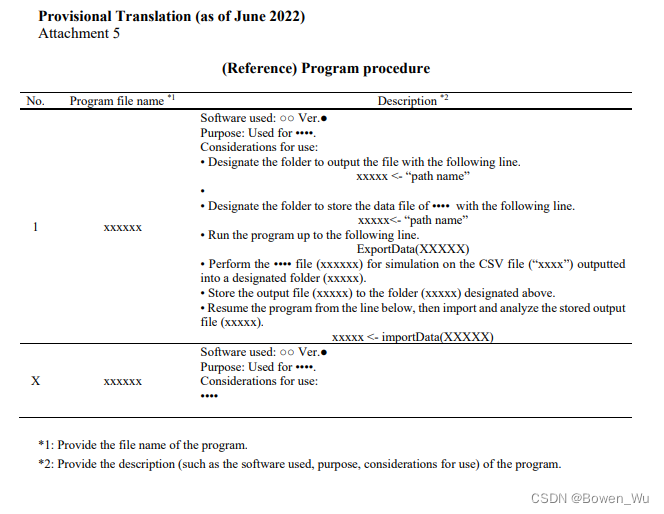
(6) Program procedures 程序流程
Please describes the detailed procedures of running the program. It must include at least the program file names and the explanation of the programs and must be created by referring to Attachment 5. If it is not necessary to perform special processes, such as designating the path name, to use the submitted program, basically, the submission of program procedures is not necessary.
请说明运行程序的详细程序。它必须至少包含程序文件名和程序说明,并且必须通过引用附件5来创建。如果不需要执行特殊过程,例如指定路径名,就可以使用提交的程序,基本上不需要提交程序程序。
4.2.2.3 Physiologically based pharmacokinetic model analysis (including simulations) 基于生理学的药代动力学模型分析(包括模拟)
Details for the submission of electronic study data on physiologically-based pharmacokinetic model analysis are as follows:
提交基于生理学的药代动力学模型分析的电子研究数据的细节如下:
(1) Files that contain information on the model structure used for the analysis, the set values of drug and physiological parameters, analysis results, and sensitivity analyses
包含用于分析的模型结构,药物和生理参数的设定值,分析结果和灵敏度分析的信息的文件
The file format is optional.
文件格式可选。
(2) Clinical study datasets, including blood concentration data 临床研究数据集,包括血浓度数据
If the datasets were created or modified to be analyzed using a specific software for PBPK model analysis, the electronic files of the created or modified datasets should be submitted in the format for the specific software [Simcyp PE Data Files (xml format), etc.]. If the datasets were not created or modified for a specific software for PBPK model analysis, the datasets can be submitted in an optional file format. When submitting clinical study datasets, including blood concentration data, dataset definition documents should be submitted [refer to 4.2.2.1 (2)] as needed.
如果数据集是使用特定软件进行PBPK模型分析创建或修改的,则创建或修改的数据集的电子文件应以特定软件的格式提交[Simcyp PE数据文件(xml格式)等]。如果未为用于 PBPK 模型分析的特定软件创建或修改数据集,则可以以可选文件格式提交数据集。在提交临床研究数据集(包括血浓度数据)时,应根据需要提交数据集定义文件[参见4.2.2.1(2)]。
5. Relationship between electronic study data and eCTD for new drug applications 电子研究数据与eCTD在新药应用中的应用关系
(略)
6. Others 其他
For products that require the submission of electronic study data but do not necessarily require the attachment to a new drug application to be a CTD, such attachments to the new drug application may be submitted electronically by the method specified in 3. In such a case, the standard electronic specification of the attachments to the new drug application will be provided separately.
对于需要提交电子研究数据但不一定要求新药申请附件为CTD的产品,新药申请的附件可以通过3中规定的方法以电子方式提交。在这种情况下,将单独提供新药申请附件的标准电子规范。
附件1

附件2

附件3

附件4

附件5